MRL means “Maximum Residue Level". It is the maximum level of pesticide residue that is legally permitted in or on a given food or feed product.
In the EU, MRLs are set in Regulation (EC) No 396/2005 ensuring consumer health protection. An MRL is set based on the level of residue expected from a Good Agricultural Practice (GAP), i.e., a nationally recommended, authorised or registered safe uses of plant protection products. The GAP can be authorised either:
- in an EU Member State
- in a non-EU country:
- an application for an MRL can be submitted to the EU for an "import tolerance", or
- an MRL dossier can be submitted to the Codex Alimentarius (Codex MRL, “CXL”).
The same MRLs apply to all food and feed products placed on the EU market, whether they are produced in the EU or imported from non-EU countries.
MRL setting in the EU is a robust system based on science a with separate risk assessment and risk management processes.
Applicants, who can be producers of plant protection products, farmers, importers, EU or non-EU countries, must submit a required set of data to an EU Member State to request the setting of an MRL. The following information is required:
- Details of the GAP, which is the use of the pesticide on the crop, including: quantity, frequency, growth stage of the plant, etc;
- Experimental data on the expected residues on food/feed when the pesticide is applied according to the GAP (also called residue field trials);
Toxicological data of the pesticide. Chronic toxicity is expressed as percentage of the Acceptable Daily Intake (ADI), acute toxicity as percentage of the Acute Reference Dose (ARfD).
The risk assessment is performed both by the EU Member State where the application is submitted and by the European Food Safety Authority (EFSA). The evaluating EU Member State assesses the information and submits an evaluation report to EFSA. As part of its risk assessment to confirm the safety of the requested MRL, EFSA verifies that this MRL is safe for all European consumer groups, including vulnerable groups such as babies, children and vegetarians. EFSA compares the dietary intake of residues through all food that may be treated with that pesticide with the ADI and the ARfD of the substance.
- When a risk is established for any consumer group, the MRL application will be rejected, and the pesticide will not be allowed to be used in those conditions.
If there is no health risk, EFSA recommends an MRL based on the value calculated via the MRL calculator of the OECD (Organisation for Economic Co-operation and Development). The “ALARA” approach is followed, meaning that MRLs are set at a level “as low as reasonably achievable”.
For crops on which the pesticide is not used or does not leave detectable residues, the MRL is set at the Limit of Quantification (LOQ), which is the technical zero of the analytical technique for that substance on that food/feed. In Regulation (EC) 396/2005, it is referred to as “Limit of Determination” (LOD).
EFSA publishes its risk assessment as Reasoned Opinions in the EFSA Journal. More detailed information can be found in the “Technical Guidelines on MRL setting procedure in accordance with Articles 6 to 11 of Regulation (EC) 396/2005 and Article 8 of Regulation (EC) 1107/2009”.
Following EFSA’s opinion, the European Commission prepares a draft Regulation setting the new MRL(s) and a discussion is held with the EU Member States in the Standing Committee for Plants, Animals, Food and Feed (SCoPAFF). EU Member States are invited to vote on the draft Regulation, which follows subsequently the “Regulatory Procedure with Scrutiny” (see Question 8).
From the date of the vote by the EU Member States in the SCoPAFF, in case the vote results in a favourable opinion and the scrutiny does not result in an objection, the approximate timeline is the following.
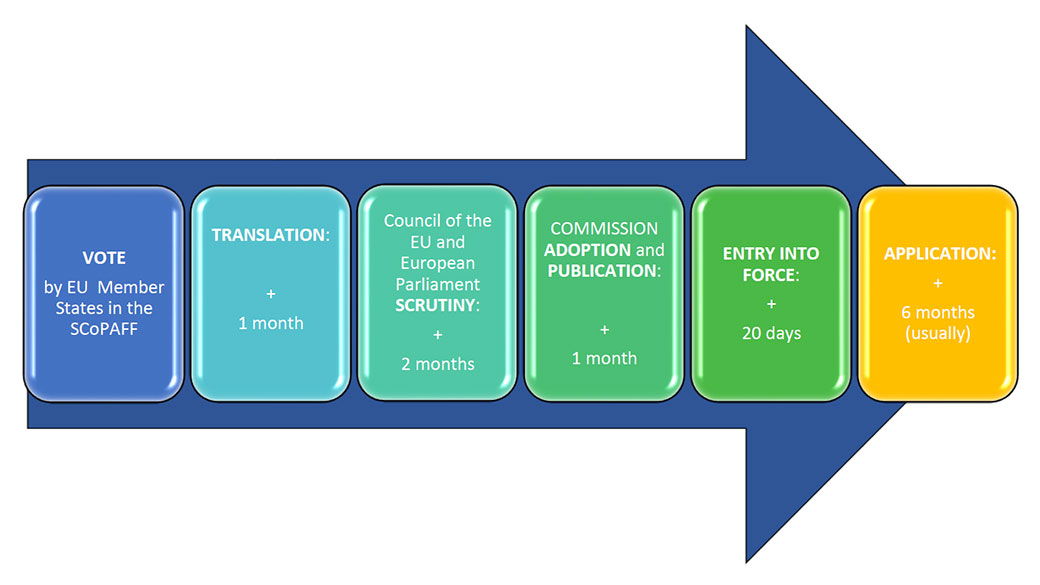
- VOTE by EU Member States in the SCoPAFF
- TRANSLATION: + 1 month
- Council of the EU and European Parliament SCRUTINY: + 2 months
- COMMISSION ADOPTION and PUBLICATION: + 1 month
- ENTRY INTO FORCE: + 20 days
- APPLICATION: + 6 months (usually)
The application date of a Regulation setting new MRLs is the date at which the new MRLs start to apply.
- When the new MRL is higher than the previous value: the application date is as soon as possible, usually at the time of the entry into force, i.e., 20 days after publication of the Regulation in the Official Journal of the EU. This is approximately 5 months after the vote by Member States in the SCoPAFF.
When the new MRL is lower than the previous value: the application date is deferred, meaning that an additional period is given after the entry into force. This is to allow EU Member States, non-EU countries and food business operators to prepare themselves for the application of the new, lower, MRL. The deferred application period is usually 6 months after the entry into force, in line with
WTO recommendation. It can be shorter in exceptional cases, for example in case of health concerns (e.g., phosmet, oxamyl).
A transitional measure is when products compliant with the “old” MRL and placed on the market in the EU before the application date are allowed to remain on the market even after the application date of the new MRL. This applies in the same way to products produced in the EU or imported in the EU. If a transitional measure is granted, it is explicitly written in the articles of the Regulation setting the new MRL. In case of any health concerns with the “old” MRLs, no transitional measures are granted.
The main objective of Regulation (EC) No 396/2005 is to ensure consumer protection and public health. MRLs can only be established if EFSA has confirmed that they are safe for consumers. This requires that a minimum of supporting data is provided in line with the EU data requirements, allowing EFSA to reach a conclusion on the safety of the MRL. If this supporting evidence is lacking or insufficient so that EFSA cannot conclude that the MRL is safe, that MRL cannot be set.
The burden of providing the relevant information and data is on the applicants. Risk assessment is carried out in an independent manner and applicants cannot influence its outcome.
Article 5.7 of the SPS Agreement indicates that in cases where relevant scientific evidence is insufficient, a WTO Member may provisionally adopt sanitary or phytosanitary measures based on available pertinent information. The precautionary principle allows risk managers to take SPS measures when the possibility of harmful effects on health has been identified based on available scientific evidence and such measures are necessary to ensure a high level of health protection in the EU.
MRL setting in the EU is based on a scientific risk assessment carried out by both an evaluating Member State and the EFSA and using the most up to date science and evidence available. Obviously, science is under continuous development with new data and risk assessment methodologies becoming available. Therefore, the EU has procedures in place to review any measure at any moment, if necessary. This can be done based on an application submitted by an applicant and supported by the necessary evidence or triggered by an EU institution or a Member State.
Certain review processes are set up with a clear schedule and work programme. For example, the EU is currently carrying out a comprehensive review programme for
existing MRLs under Article 12 of Regulation (EC) 396/2005. Details of the respective EFSA risk assessments can be found on the EFSA website. In addition, EFSA publishes in its website on a quarterly basis its detailed work programme and indicative time schedule for the substances to be reviewed.
When there is no specific MRL set in Regulation (EC) 396/2005 for a given active substance in/on a given food or feed product, a default level of 0.01 mg/kg applies.
This approach is protective of consumer health and ensures legal certainty.
Many non-EU countries have also a default level, for example India and Japan (same value as the EU), Canada and New Zealand (0.1 mg/kg); while others do not have a default level (Australia, USA).
In cases when EFSA concludes that an MRL is safe, the European Commission, in accordance with the EU legislation and with its obligations under the WTO, in particular the SPS Agreement, prepares a draft Regulation to set the relevant MRL.
Under the EU legislation on the control by Member States of the Commission's exercise of powers conferred on it by the Council of the EU, the draft Regulation must be presented for vote to EU Member States representatives in a regulatory committee, the Standing Committee for Plants, Animals, Food and Feed (SCoPAFF).
- If a qualified majority is reached in favour, the draft Regulation is translated in all EU official languages and submitted for scrutiny to the Council of the EU and the European Parliament under the “Regulatory Procedure with Scrutiny”. If no objections are raised, the Commission adopts the Regulation, which is then published in the Official Journal. The Regulation enters into force 20 days after publication and the new MRLs apply from the date set in the Regulation, usually 6 months after the entry into force (see Question 4).
- If a qualified majority is reached against, the draft Regulation may not be adopted.
- If a qualified majority is not reached in favour or against, the Commission submits the draft Regulation to the Council which has to act within 1 month by either opposing it or by envisaging to adopt it. In both cases qualified majority must be obtained. If the Council opposes the draft Regulation with a qualified majority, the Commission may not adopt it. If the Council does not act within the 1-month period, the Commission must submit the draft Regulation for scrutiny to the European Parliament who may either not act or oppose the measure. If opposed, the Commission may not adopt it.
The EU MRL-setting process is transparent and predictable.
The full list of MRLs that are established in the EU are publicly accessible via the EU Pesticide Database. The database also includes information on those MRLs for which legislation has already been adopted but which is not yet applicable.
Several early-warning mechanisms have been put in place to alert on upcoming changes in the preparation phase of any draft Regulation setting new MRLs:
- Ongoing EU discussions on draft Regulations are published in the EU comitology register and information can be found in the summary reports of the SCoPAFF.
- The EU submits for consultation with trading partners through the SPS Agreement and/or the TBT Agreement of the WTO all draft Regulations regarding active substances and regarding MRLs, several months before they are voted and adopted. The notification of a draft decision of non-renewal of approval of an active substance already gives information on the likelihood of a subsequent modification of the EU MRLs, on average 3 to 4 years before the new MRLs would apply.
- Risk assessments for active substances and MRLs are published on the EFSA website. In case of the systematic review of MRLs under Article 12 of Regulation (EC) 396/2005, the publication is roughly 2 years before any new MRLs would apply. EFSA also publishes a quarterly updated overview of substances that are for review.
Controls of pesticide residues and enforcement of MRLs are the responsibility of EU Member States. General requirements for controls of food and feed are set out in Regulation (EU) No 2017/625 (Official Controls Regulation, ‘OCR’). As specified in Regulation (EC) No 396/2005, EU Member States carry out official controls on pesticides residues, in accordance with the relevant provisions of the OCR, monitor compliance with existing EU MRLs and assess consumer exposure to pesticide residues.
Actions to be taken by EU Member States in the case of non-compliances are laid down in Article 138 of the OCR. In case of established non-compliance, competent authorities must take any measure they deem appropriate to ensure compliance with the EU agri- food chain legislation, including, but not limited to:
- order the treatments of goods;
- restrict or prohibit the placing on the market, the movement, the entry into the EU or the export of goods;
- prohibit their return to the EU Member State of dispatch or order their return to the EU Member State of dispatch;
order the recall, withdrawal, removal and destruction of goods, authorising, where appropriate, the use of the goods for purposes other than those for which they were originally intended.
The European Commission carries out controls in EU Member States on compliance with the EU legislation on food and feed and in third countries to verify compliance or equivalence of third-country legislation and systems. The applicable requirements are set out in Articles 116-123 of the OCR.
There is no difference of MRL enforcement between products imported in the EU and produced in the EU.
In case of a Regulation setting new MRLs, the applicable MRLs depend on whether a transitional measure is granted (see Question 4):
- If no transition measure is granted, MRLs are enforced based on the MRLs in effect on the date of application of the Regulation setting the MRLs.
If a transition measure is granted, MRLs are enforced based on the MRLs in effect on the date of the placing the food or feed on the market in the EU. The definition of “placing on the market” is set in Article 3 of Regulation (EC) No 178/2002 (the General Food Law), to which Article 3(1) of Regulation (EC) No 396/2005 refers. “Placing on the market” means the “holding of food or feed for the purpose of sale, including offering for sale or any other form of transfer, whether free of charge or not, and the sale, distribution, and other forms of transfer themselves”.
As per this definition, conditions have to be fulfilled for a food or feed to be considered as already “placed on the market”:
- The food or feed must have obtained its final characteristics, i.e., any process that gives it its final characteristics must be finalised. For example, storage of wine for maturation purposes is not placing on the market.
- The holding of the food or feed must be for the purpose of sale, including offering for sale, for example on a supermarket shelf or in a storage facility of a supermarket. If the product is stored for other purposes, for example for exports, it is not placing on the market.
Regulation (EC) No 396/2005 requires the establishment of both EU-coordinated and national control programmes:
- An EU-coordinated multi-annual control programme (‘MACP’) that defines the pesticide/product combinations to be monitored by all EU Member States and European Economic Area (EEA) countries and the minimum number of samples to be taken for each product. EU Member States must submit the results of the analysis of samples tested.
- Multi-annual national control programmes (‘MANCPs’): these programmes are updated every year and are consistent with the MACP. They indicate the products to be sampled, the number of samples to be taken, the pesticides to be analysed and the criteria used for drawing up the programmes. EU Member States define the scope of national control programmes focussing on certain products, which are expected to have a higher probability to contain residues in concentrations exceeding the residue limits, or on products that may pose risks for consumer safety.
The MACP and the MANCPs target food both imported and produced in the EU.
The data collected is transmitted to EFSA, which issues every year a comprehensive annual report on pesticides residues found in food products. The annual reports are published on the EFSA website.
According to the annual report of EFSA issued in 2024, more than 110 000 samples were collected and analysed in 2022 for over 750 different pesticides. It showed that 51% of products were free of quantifiable residues altogether and 47% contained traces not exceeding the MRL. 3.7% of the samples had residues above the MRL, out of which, 2.2% were non-compliant, meaning that the results exceeded the MRL taking into account the analytical uncertainty, and enforcement actions were considered. These figures have been roughly stable over the past 10 years, showing that the control systems work well. EFSA additionally confirmed via a comprehensive consumer exposure assessment that there is no health risk for consumers.
The EU is committed to engaging actively with all its trading partners. It is conducting constructive dialogs in multilateral fora as well as bilaterally. It aims to achieve a transition towards a more sustainable use of pesticides together and promote alternative products, while avoiding disruptions in trade.
Its actions comprise:
- International cooperation in international fora (WTO SPS Committee, Codex Alimentarius),
- Inclusion of Chapters on Sustainable Food Systems in trade agreements,
- EU-funded programmes to assist non-EU countries,
Specific seminars for information of non-EU countries.
Trading partners can actively participate and provide input during the review process of EU MRLs, following the detailed guidance given to WTO Members16.
The EU funds several programmes to assist non-EU countries, in particular developing countries, to comply with EU legislation and to build capacity and knowledge, such as the Agrinfo programme (managed by COLEAD, the Committee Linking Entrepreneurship Agriculture and Development), further the existing Fit for Market and Plantwise Plus programmes to name only a few examples. The EU also organises specific training courses related to plant health, integrated pest management and food safety in relation to pesticide residues.
16 G/SPS/GEN/1494/Rev.2
The EU funds several specific programmes that can help farmers in non-EU countries to find suitable alternatives, and to comply with EU regulatory requirements, notably:
- “Fit for market+” programme by the Europe-Africa-Caribbean-Pacific Liaison Committee (COLEAD). “Fit for market+” helps farmers to find alternative pesticides, allows smallholder farmers, producer groups, farmer organisations, and small and medium enterprises to access international and domestic fruit and vegetable markets by complying with the SPS standards and market requirements, in a sustainable framework.
- “Plantwise+” programme by the Centre for Agricultural Bioscience International (CABI). It aims to improve farmers’ yields and incomes while reducing the use of toxic pesticides, to increase food security and improve rural livelihoods by reducing crop losses and addressing issues regarding the safe use of pesticides. Working in close partnership with relevant actors, Plantwise+ strengthens national plant health systems, enabling countries to provide farmers with the knowledge they need to lose less of what they grow.
- “Better Training for Safer Food” (BTSF) by the Directorate-General for Health and Food Safety of the European Commission (DG SANTE). BTSF are training initiatives for issues related to food and feed safety, including pesticides residues and integrated pest management, as well as animal health and welfare, and plant health rules. BTSF aims to ensure safety of food imports from non-EU countries on the EU market, harmonisation of control procedures between EU and non-EU partners, and fair trade with non-EU countries and in particular developing countries.
The EU receives input from its trading partners via several channels:
- Submission of import tolerance requests to set an EU MRL based on a use in a non-EU country (see next question).
- In the context of the systematic review of EU MRLs under Article 12 of Regulation (EC) No 396/2005, early submission of additional data before the risk assessment is carried out by EFSA. Non-EU countries should first contact the Rapporteur Member State in charge of the active substance evaluation. During the WTO/SPS consultation procedure, all trade concerns raised by trading partners are duly considered and taken into account by the EU. The EU studies carefully the comments received and discusses them with the EU Member States at the meetings of the SCoPAFF before any draft Regulation is adopted.
The EU provides detailed replies in writing explaining the rationale for its proposals and decisions and keeps a transparent approach with its trading partners. When appropriate, the EU has taken action by modifying some of its proposals to respond to issues raised by other WTO member countries.
For example, Regulation (EU) 2023/1753 updating MRLs for pyriproxyfen was adopted on 11 September 2023. The draft Regulation had been notified to the WTO-SPS Committee on 23 February 2023 in G/SPS/N/EU/618. A request was received not to lower the MRL for banana, which was at a safe level, pending the submission of a full import tolerance request. The request was supported by Colombia and Ecuador. As concrete data from a first residue trial was submitted with specific plans for further generation of data, along with support from the producing countries, the EU decided to grant the request and maintain the MRL for bananas, pending the submission of an import tolerance request.
The EU acknowledges that non-EU countries may face production conditions and pest pressures different from those in mainland Europe. Therefore, the EU legislation provides for the possibility to set MRLs to meet the needs of international trade and requires the consideration of good agricultural practices authorised in non-EU countries as well as of MRLs set by the Codex Alimentarius Commission.
Applications for setting a MRL undergo a risk assessment, described in Question 2. The same criteria and process apply to applications based on a use in the EU and to those based on a use in a non-EU country. The evaluation of an import tolerance takes on average around 24 months, but this can vary depending on the complexity of the assessment.
Regulation (EC) 396/2005 stipulates that MRLs set at international level by the Codex Alimentarius Commission (CAC) should be considered when EU MRLs are being set. Thus, EU MRLs are regularly aligned with Codex MRLs (CXLs), provided that:
- the EU sets MRLs for the commodity under consideration; and
the current EU MRL is lower than the CXL (otherwise the current EU MRL already covers for the CXL).
The EU considers that CXLs are an important tool to facilitate international trade and a lot of thought has been given to a systematic approach to assess and implement them. Newly proposed CXLs are assessed by EFSA in an annual scientific report, which is publicly available in the EFSA journal. CXLs are generally taken over in EU legislation, which generally is in conformity with them, unless the EU raised a concern ("reservation") at the meeting of the Codex Committee on Pesticide Residues (CCPR). The EU introduces reservations to the advancement of proposed CXLs:
- if the proposed CXL is not considered to be safe for European consumers by EFSA, and/or
- if toxicological data are not available at EU level or are available but not yet assessed at EU level, and/or
- if the proposed CXLs are not sufficiently supported by the required data, and/or
- if the CXL is not acceptable to the EU with respect to areas such as supporting data, and extrapolations, as well as environmental issues of global nature (such as the decline of pollinators or the accumulation of persistent bioaccumulative and toxic substances in the environment).
The purpose of raising reservations is to increase transparency and predictability in international trade. The EU is one of the very few or the only Codex member openly and transparently raising reservations and communicating to Codex membership every time when not in a position to adopt a new CXL, providing scientific reasons for the reservations and consequent non-alignment.
Over the period 2018-2023, the EU has implemented around 50% of the CXLs that were proposed for adoption by Codex members.
The CXLs are transposed into EU legislation by dedicated Regulations drafted on a yearly basis after the meeting of the Codex Alimentarius Commission (CAC) usually in November. A WTO SPS notification is additionally issued for transparency.
Abbreviations
- CJEU: Court of Justice of the European Union
- CXL: Codex Maximum Residue Limit EFSA: European Food Safety Authority EU: European Union
- FAO: Food and Agriculture Organization of the United Nations
- GAP: Good Agricultural Practice
- JMPR: Joint Meeting on Pesticide Residues
- LOQ: Limit of Quantification
- MRL: Maximum Residue Level
- SCoPAFF: Standing Committee on Plants, Animals Food and Feed
- SPS Committee: WTO Committee on Sanitary and Phytosanitary measures
- TBT Committee: WTO Committee on Technical Barriers to Trade
- WHO: World Health Organization
- WTO: World Trade Organization
Resources
Information on MRLs
- MRL Regulation: Regulation (EC) 396/2005
- PPP Regulation: Regulation (EU) 1107/2009
- Official Control Regulation: Regulation (EU) 2017/625
- General Food Law: Regulation (EC) No 178/2002
- Legal texts (Official Journal)
- Technical guidelines on MRLs in the EU:
- Guidance document on MRL setting procedure for MRL applications submitted as of 27 March 2021: SANTE/2015/10595 Rev. 6.1
- EU pesticides database:
- SPS Note to non-EU countries about the ongoing systematic MRL review process under Article 12 of Regulation (EC) 396/2005:
- EFSA overview of the MRL review progress under Article 12 of Regulation (EC) No 396/2005.
- Public Commission Comitology Register (Draft and final legal texts)
- Summary reports of regular meetings of the Standing Committee of Plants, Animals, Food and Feed (SCoPAFF), section Phytopharmaceuticals, Pesticides Residues:
- Refit evaluation
